The Balancing Equations Gizmo Answer Key unlocks the secrets of chemical equilibrium, providing a comprehensive guide to understanding the fundamental principles of balancing equations. This interactive resource empowers students and educators alike with a deep dive into the concepts and applications of chemical reactions.
Through step-by-step instructions, the Gizmo Answer Key demystifies the process of balancing equations, equipping users with the knowledge and skills to solve complex chemical problems with precision and confidence.
Balancing Equations Gizmo Introduction

The Balancing Equations Gizmo is an interactive simulation that helps students understand the concept of balancing chemical equations. A chemical equation is a symbolic representation of a chemical reaction, and it must be balanced to ensure that the number of atoms of each element is the same on both sides of the equation.
paragraphThe Gizmo provides a visual representation of a chemical reaction, and it allows students to manipulate the coefficients of the reactants and products to balance the equation. The Gizmo also provides feedback on the student’s work, and it can help students identify and correct errors.
Key Features and Functionality
- Interactive simulation of a chemical reaction
- Allows students to manipulate the coefficients of the reactants and products
- Provides feedback on the student’s work
- Can help students identify and correct errors
Procedure and Methods
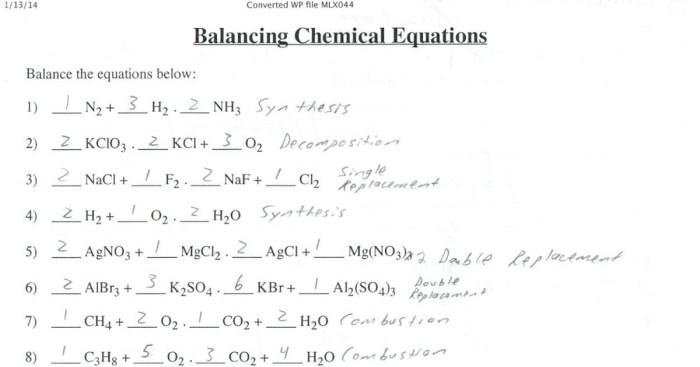
The Balancing Equations Gizmo allows users to explore the concept of balancing chemical equations. To use the Gizmo, follow these steps:
1. Select the “Reactants” and “Products” tabs to view the chemical formulas of the reactants and products in the reaction. 2. Use the “Add” and “Remove” buttons to adjust the coefficients of the reactants and products. 3. Click the “Balance” button to have the Gizmo automatically balance the equation.
4. Observe the changes in the coefficients and the overall charge of the equation.
There are several methods and techniques for balancing equations. One common method is the half-reaction method, which involves balancing the oxidation and reduction half-reactions separately. Another method is the oxidation number method, which involves assigning oxidation numbers to the atoms in the reactants and products and then adjusting the coefficients to balance the charges.
Examples of Balanced and Unbalanced Equations
A balanced equation has the same number of atoms of each element on both sides of the equation. An unbalanced equation has a different number of atoms of one or more elements on the two sides of the equation.
Here is an example of a balanced equation:
“`
H2+ O 2→ 2H 2O
“`
Here is an example of an unbalanced equation:
“`CH 4+ 2O 2→ CO 2+ 2H 2O“`
Chemical Reactions: Balancing Equations Gizmo Answer Key
Chemical reactions are processes in which atoms or molecules are rearranged to form new substances. They are represented by chemical equations, which show the reactants (the initial substances) on the left side and the products (the final substances) on the right side.
The Gizmo can be used to represent and analyze chemical reactions by allowing you to drag and drop atoms and molecules to create reactants and products. You can then balance the equation by adjusting the coefficients in front of each reactant and product.
Types of Chemical Reactions
There are many different types of chemical reactions, but some of the most common include:
- Combination reactions: Two or more substances combine to form a single product.
- Decomposition reactions: A single substance breaks down into two or more products.
- Single-replacement reactions: One element replaces another element in a compound.
- Double-replacement reactions: Two compounds exchange ions to form two new compounds.
Balancing Equations
Balancing equations is a crucial step in chemistry as it ensures that the number of atoms of each element on the reactant side of a chemical equation equals the number of atoms of that element on the product side. This is essential for understanding the stoichiometry of a reaction, which is the quantitative relationship between the reactants and products involved.
There are several methods for balancing equations, including:
Inspection Method, Balancing equations gizmo answer key
- Start by identifying the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the stoichiometric coefficients (the numbers in front of each chemical formula) in front of each reactant and product to balance the number of atoms of each element.
- Check the equation to ensure that it is balanced.
Half-Reaction Method
- This method is used for redox reactions, which involve the transfer of electrons.
- The reaction is divided into two half-reactions, one for oxidation and one for reduction.
- Each half-reaction is balanced separately for mass and charge.
- The two half-reactions are then combined to form the overall balanced equation.
Matrix Method
- This method is used for complex reactions involving multiple reactants and products.
- A matrix is set up with the stoichiometric coefficients of each reactant and product as the elements of the matrix.
- The matrix is then manipulated using mathematical operations to find the values of the stoichiometric coefficients that balance the equation.
Examples of Balancing Equations Using the Gizmo
The Gizmo allows users to practice balancing equations using various methods. For example, users can:
- Drag and drop atoms to create molecules and balance equations.
- Use the “Balance” button to automatically balance equations.
- View step-by-step instructions on how to balance equations using different methods.
By using the Gizmo, students can gain a better understanding of the importance of balancing equations and develop their skills in using different balancing methods.
Applications and Real-World Examples
Balancing chemical equations is a fundamental skill in chemistry and various other fields. It enables scientists, engineers, and medical professionals to understand and predict the behavior of chemical reactions accurately.
In chemistry, balancing equations is crucial for stoichiometry, which involves determining the quantitative relationships between reactants and products in a chemical reaction. This information is essential for predicting the amount of reactants and products involved, ensuring efficient and safe chemical processes.
Engineering
- In chemical engineering, balancing equations is vital for designing and optimizing chemical plants and processes. It helps engineers determine the optimal ratios of reactants to achieve desired product yields and minimize waste.
- In environmental engineering, balancing equations is used to understand and mitigate the impact of chemical reactions on the environment. For example, it helps determine the amount of pollutants released in industrial processes and develop strategies to minimize their environmental impact.
Medicine
- In medicine, balancing equations is essential for understanding the chemical reactions involved in biological processes and drug interactions. It helps researchers develop new drugs and therapies and optimize their dosage and administration.
- In clinical chemistry, balancing equations is used to analyze blood samples and other bodily fluids to diagnose and monitor various diseases. By measuring the concentrations of different chemicals in the body, doctors can gain insights into metabolic processes and identify potential health issues.
Gizmo Answer Key

The Gizmo Answer Key provides the correct answers to the exercises in the Balancing Equations Gizmo. This answer key can be used by students to check their work and by teachers to assess student understanding.
To use the answer key, students can compare their answers to the correct answers provided. If a student’s answer does not match the correct answer, they can review the steps they took to solve the problem and identify where they made a mistake.
Teachers can use the answer key to quickly and easily assess student work and identify areas where students need additional support.
Answer Key Table
| Exercise | Correct Answer |
|---|---|
| 1 | 2H2 + O2 → 2H2O |
| 2 | CH4 + 2O2 → CO2 + 2H2O |
| 3 | 2C2H6 + 7O2 → 4CO2 + 6H2O |
Assessment and Evaluation
The Balancing Equations Gizmo can be an effective tool for assessment and evaluation. It provides a dynamic and interactive environment where students can demonstrate their understanding of balancing equations. By observing students’ interactions with the Gizmo, teachers can assess their progress and identify areas where additional support is needed.
Using the Gizmo to Assess Student Understanding
Here are some suggestions for using the Gizmo to assess student understanding of balancing equations:
- Formative assessment:Use the Gizmo as a formative assessment tool to monitor student progress throughout the unit. Assign Gizmo activities as homework or classwork to track students’ understanding of balancing equations. This will allow you to identify areas where students need additional support.
- Summative assessment:The Gizmo can also be used as a summative assessment tool to evaluate student learning at the end of a unit or semester. Assign a Gizmo project that requires students to demonstrate their ability to balance equations, write chemical formulas, and predict the products of chemical reactions.
- Self-assessment:The Gizmo can also be used for self-assessment. Encourage students to use the Gizmo to practice balancing equations and assess their own understanding. This will help students to identify their strengths and weaknesses and take ownership of their learning.
FAQ Insights
What is the purpose of balancing equations?
Balancing equations ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the conservation of mass in chemical reactions.
How does the Gizmo Answer Key help in balancing equations?
The Gizmo Answer Key provides step-by-step solutions to various balancing equation exercises, allowing users to check their work and identify areas for improvement.
What are the benefits of using the Gizmo Answer Key?
The Gizmo Answer Key enhances understanding of balancing equations, promotes critical thinking skills, and provides a valuable resource for self-assessment and progress monitoring.
