Determine whether each of the preceding is exothermic or endothermic – Determining whether a chemical reaction is exothermic or endothermic is a fundamental aspect of understanding chemical processes. This guide delves into the intricacies of these reactions, providing a comprehensive exploration of their characteristics, methods of determination, and practical applications.
By examining the enthalpy changes associated with reactions, we can uncover their energetic nature and gain insights into their potential uses. From combustion reactions that release heat to endothermic reactions that require external energy input, this guide unravels the fascinating world of chemical energetics.
1. Types of Chemical Reactions: Determine Whether Each Of The Preceding Is Exothermic Or Endothermic

Chemical reactions can be classified as either exothermic or endothermic based on the energy changes that occur during the reaction.
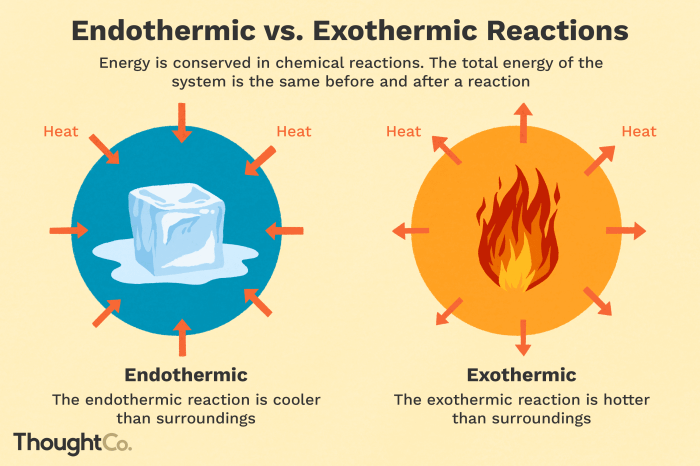
Exothermic reactionsare reactions that release energy in the form of heat, light, or sound. The products of an exothermic reaction have less energy than the reactants, and the energy released is equal to the difference in energy between the products and reactants.
Endothermic reactionsare reactions that require energy to occur. The products of an endothermic reaction have more energy than the reactants, and the energy absorbed is equal to the difference in energy between the products and reactants.
2. Enthalpy and Energy Changes

Enthalpy is a thermodynamic property that measures the total energy of a system. The change in enthalpy during a chemical reaction is equal to the heat absorbed or released by the reaction.
For an exothermic reaction, the change in enthalpy is negative because heat is released. For an endothermic reaction, the change in enthalpy is positive because heat is absorbed.
3. Methods for Determining Exothermic or Endothermic Reactions
There are several experimental methods that can be used to determine the enthalpy change of a reaction, and thus whether the reaction is exothermic or endothermic.
- Calorimetry: Calorimetry is a technique that measures the heat released or absorbed by a reaction. A calorimeter is a device that is used to measure the temperature change of a reaction. The heat released or absorbed by the reaction can be calculated from the temperature change and the heat capacity of the calorimeter.
- Bomb calorimetry: Bomb calorimetry is a type of calorimetry that is used to measure the heat released by a reaction that occurs in a closed container. The bomb calorimeter is a device that is designed to withstand the high pressures that can be generated by a reaction.
4. Applications of Determining Exothermic or Endothermic Reactions
Determining whether a reaction is exothermic or endothermic has several practical applications, including:
- Predicting the feasibility of a reaction: Exothermic reactions are more likely to occur spontaneously than endothermic reactions. This is because exothermic reactions release energy, which can be used to drive the reaction forward.
- Designing chemical processes: The enthalpy change of a reaction can be used to design chemical processes that are more efficient and less energy-intensive.
- Understanding the behavior of materials: The enthalpy change of a reaction can be used to understand the behavior of materials. For example, the enthalpy change of a phase transition can be used to determine the melting point or boiling point of a material.
5. Examples and Case Studies

The following are some examples of exothermic and endothermic reactions:
- Exothermic reactions:
- Combustion of fuels
- Neutralization of acids and bases
- Precipitation reactions
- Endothermic reactions:
- Melting of ice
- Dissolution of salts in water
- Endothermic reactions
Questions Often Asked
What is the difference between an exothermic and an endothermic reaction?
Exothermic reactions release heat into the surroundings, while endothermic reactions absorb heat from the surroundings.
How can I determine if a reaction is exothermic or endothermic?
The enthalpy change of a reaction can be measured experimentally using calorimetry. A positive enthalpy change indicates an endothermic reaction, while a negative enthalpy change indicates an exothermic reaction.
What are some examples of exothermic reactions?
Combustion reactions, such as burning wood or gasoline, are exothermic reactions that release heat.
What are some examples of endothermic reactions?
Photosynthesis, the process by which plants convert sunlight into energy, is an endothermic reaction that requires energy input.
